Embark on a scientific odyssey with gizmo temperature and particle motion answers, where we unravel the intricate relationship between these fundamental concepts. This discourse delves into the heart of matter, exploring how temperature governs the behavior of particles, influencing their kinetic energy, diffusion, chemical reactions, phase transitions, and thermal expansion.
As we navigate this intellectual landscape, we will uncover the profound impact of temperature on the microscopic world, shedding light on the dynamic nature of matter and its response to thermal stimuli.
Gizmo Temperature and Particle Motion
Temperature is a measure of the average kinetic energy of the particles in a substance. The higher the temperature, the faster the particles move. This is because the particles have more energy at higher temperatures.
The kinetic energy of a particle is the energy of motion. It is determined by the mass of the particle and its velocity. The faster a particle moves, the more kinetic energy it has. The more massive a particle is, the more kinetic energy it has for a given velocity.
Temperature affects the kinetic energy of particles in several ways. First, temperature affects the average speed of particles. At higher temperatures, particles move faster on average. Second, temperature affects the distribution of speeds of particles. At higher temperatures, there are more particles with high speeds.
Third, temperature affects the number of collisions between particles. At higher temperatures, there are more collisions between particles.
Examples of How Temperature Changes Affect Particle Motion
- When a solid is heated, the particles in the solid gain kinetic energy and move faster. This causes the solid to expand.
- When a liquid is heated, the particles in the liquid gain kinetic energy and move faster. This causes the liquid to expand and become less dense.
- When a gas is heated, the particles in the gas gain kinetic energy and move faster. This causes the gas to expand and become less dense.
Gizmo Temperature and Diffusion

Diffusion is the movement of particles from an area of high concentration to an area of low concentration. The rate of diffusion is determined by the temperature, the concentration gradient, and the size of the particles.
Temperature affects the rate of diffusion in several ways. First, temperature affects the kinetic energy of particles. At higher temperatures, particles move faster. This increases the rate of diffusion.
Second, temperature affects the concentration gradient. At higher temperatures, the particles are more evenly distributed. This decreases the concentration gradient and decreases the rate of diffusion.
Third, temperature affects the size of the particles. At higher temperatures, the particles are more likely to break apart. This decreases the size of the particles and increases the rate of diffusion.
Examples of How Temperature Changes Affect the Rate of Diffusion, Gizmo temperature and particle motion answers
- When a solid is heated, the particles in the solid gain kinetic energy and move faster. This increases the rate of diffusion.
- When a liquid is heated, the particles in the liquid gain kinetic energy and move faster. This increases the rate of diffusion.
- When a gas is heated, the particles in the gas gain kinetic energy and move faster. This increases the rate of diffusion.
Gizmo Temperature and Chemical Reactions
Chemical reactions are the processes by which atoms and molecules are rearranged to form new substances. The rate of a chemical reaction is determined by the temperature, the concentration of the reactants, and the presence of a catalyst.
Temperature affects the rate of a chemical reaction in several ways. First, temperature affects the kinetic energy of the reactants. At higher temperatures, the reactants have more kinetic energy and are more likely to collide with each other. This increases the rate of the reaction.
Second, temperature affects the activation energy of the reaction. The activation energy is the minimum amount of energy that the reactants must have in order to react. At higher temperatures, the reactants are more likely to have enough energy to overcome the activation energy.
This increases the rate of the reaction.
Examples of How Temperature Changes Affect the Rate of Chemical Reactions
- When a solid is heated, the particles in the solid gain kinetic energy and move faster. This increases the rate of chemical reactions.
- When a liquid is heated, the particles in the liquid gain kinetic energy and move faster. This increases the rate of chemical reactions.
- When a gas is heated, the particles in the gas gain kinetic energy and move faster. This increases the rate of chemical reactions.
Gizmo Temperature and Phase Transitions

Phase transitions are the changes in the physical state of a substance. The three main phase transitions are melting, freezing, and boiling. Melting is the change from a solid to a liquid. Freezing is the change from a liquid to a solid.
Boiling is the change from a liquid to a gas.
Temperature affects phase transitions in several ways. First, temperature affects the kinetic energy of the particles in the substance. At higher temperatures, the particles have more kinetic energy and are more likely to overcome the forces that hold them in their current phase.
Second, temperature affects the intermolecular forces between the particles in the substance. At higher temperatures, the intermolecular forces are weaker and the particles are more likely to move around. This makes it easier for the particles to change phase.
Examples of How Temperature Changes Affect Phase Transitions
- When a solid is heated, the particles in the solid gain kinetic energy and move faster. This causes the solid to melt.
- When a liquid is heated, the particles in the liquid gain kinetic energy and move faster. This causes the liquid to boil.
- When a gas is cooled, the particles in the gas lose kinetic energy and move slower. This causes the gas to condense.
Gizmo Temperature and Thermal Expansion
Thermal expansion is the increase in the volume of a substance when its temperature is increased. The coefficient of thermal expansion is a measure of the amount of expansion that occurs for a given change in temperature.
Temperature affects thermal expansion in several ways. First, temperature affects the kinetic energy of the particles in the substance. At higher temperatures, the particles have more kinetic energy and are more likely to move around. This causes the substance to expand.
Second, temperature affects the intermolecular forces between the particles in the substance. At higher temperatures, the intermolecular forces are weaker and the particles are more likely to move apart. This also causes the substance to expand.
Examples of How Temperature Changes Affect Thermal Expansion
- When a solid is heated, the particles in the solid gain kinetic energy and move faster. This causes the solid to expand.
- When a liquid is heated, the particles in the liquid gain kinetic energy and move faster. This causes the liquid to expand.
- When a gas is heated, the particles in the gas gain kinetic energy and move faster. This causes the gas to expand.
Question Bank: Gizmo Temperature And Particle Motion Answers
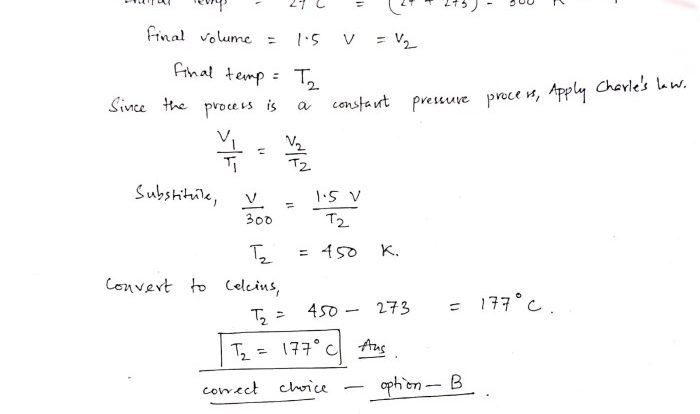
What is the relationship between temperature and particle motion?
Temperature is directly proportional to the average kinetic energy of particles. As temperature increases, the particles gain more energy and move faster.
How does temperature affect the rate of diffusion?
Temperature increases the rate of diffusion. As temperature increases, the particles have more energy and are more likely to overcome the energy barrier that prevents them from moving.
How does temperature affect the rate of chemical reactions?
Temperature increases the rate of chemical reactions. As temperature increases, the particles have more energy and are more likely to collide with each other with enough energy to react.