Embarking on the fascinating realm of accuracy and precision worksheet chemistry, this comprehensive guide delves into the intricacies of these fundamental concepts, empowering you with the knowledge and tools to excel in the field.
Accuracy and precision, the cornerstones of scientific measurement, are meticulously defined and differentiated, providing a solid foundation for understanding their significance.
Introduction
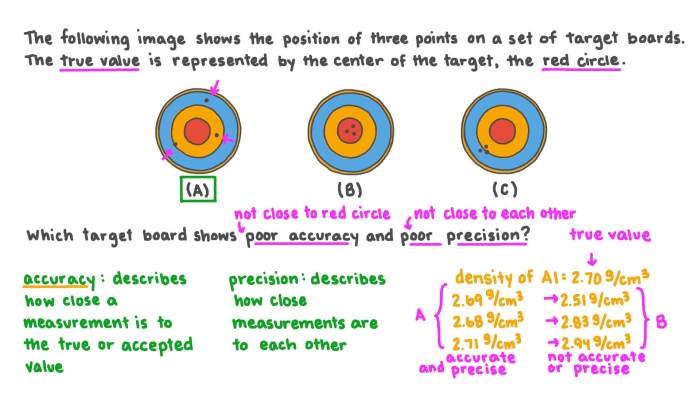
In chemistry, accuracy and precision are two important concepts that describe the quality of measurements.
Accuracy refers to how close a measurement is to the true value, while precision refers to how consistent a set of measurements is with each other.
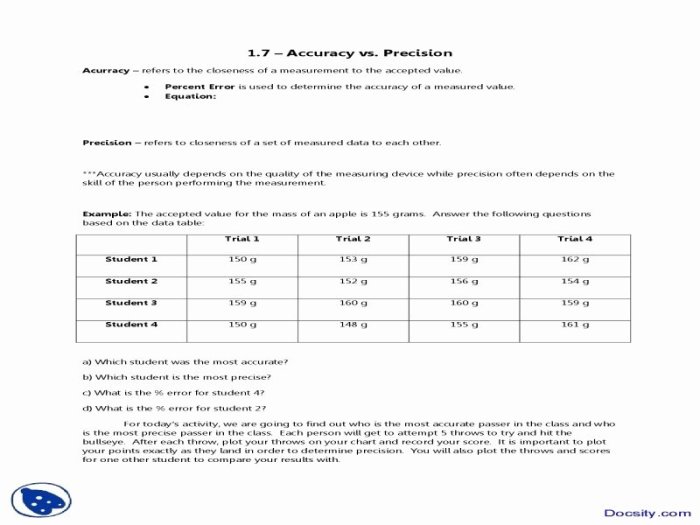
Accuracy and Precision Worksheet: Accuracy And Precision Worksheet Chemistry
An accuracy and precision worksheet can be used to calculate the accuracy and precision of a set of measurements.
To use the worksheet, first, enter the true value of the measurement and the set of measurements that were made.
The worksheet will then calculate the accuracy and precision of the measurements.
Factors Affecting Accuracy and Precision
Several factors can affect the accuracy and precision of measurements.
Factors Affecting Accuracy
- The quality of the measuring equipment
- The skill of the person making the measurements
- The environmental conditions
Factors Affecting Precision
- The number of measurements that are made
- The variability of the measurement process
- The random error
Improving Accuracy and Precision
There are several methods that can be used to improve the accuracy and precision of measurements.
Improving Accuracy
- Use high-quality measuring equipment
- Calibrate the measuring equipment regularly
- Follow the manufacturer’s instructions for using the measuring equipment
Improving Precision, Accuracy and precision worksheet chemistry
- Make multiple measurements
- Reduce the variability of the measurement process
- Minimize the random error
Applications of Accuracy and Precision
Accuracy and precision are important concepts in chemistry because they allow us to make reliable measurements.
Accuracy is important for ensuring that our measurements are close to the true value, while precision is important for ensuring that our measurements are consistent with each other.
Accuracy and precision are used in a variety of applications in chemistry, including:
- Analytical chemistry
- Physical chemistry
- Organic chemistry
- Biochemistry
Accuracy and precision are essential for ensuring the quality of our measurements and the reliability of our conclusions.
FAQ Explained
What is the difference between accuracy and precision?
Accuracy refers to how close a measurement is to the true value, while precision refers to how consistent a series of measurements are.
How can I improve the accuracy of my measurements?
Use calibrated equipment, follow proper procedures, and take multiple measurements to minimize errors.
How can I improve the precision of my measurements?
Use high-quality equipment, control variables, and repeat measurements to reduce random errors.