A gas has a pressure of 0.370 atm at 50.0 – this seemingly simple statement holds profound implications in various scientific and practical domains. Delving into the intricacies of pressure conversion, gas laws, units of measurement, and real-world applications, this exploration unveils the multifaceted nature of gas pressure and its impact on our understanding of the physical world.
The ideal gas law (PV = nRT) serves as a cornerstone in comprehending the behavior of gases under varying conditions. By examining the relationship between pressure, volume, temperature, and the number of moles, we gain insights into how gases respond to changes in their environment.
Furthermore, the precise measurement of pressure using appropriate units and devices is crucial for ensuring accuracy and reliability in scientific investigations and industrial processes.
Pressure Measurement

Pressure, a fundamental physical quantity, measures the force exerted per unit area. Understanding pressure is crucial in various scientific disciplines and real-world applications.
Pressure Conversion, A gas has a pressure of 0.370 atm at 50.0
Pressure can be expressed in different units. Common units include:
- Atmospheres (atm)
- Kilopascals (kPa)
- Millimeters of mercury (mmHg)
- Pounds per square inch (psi)
The following table provides conversion factors:
| Unit | Conversion Factor |
|---|---|
| kPa | 101.325 |
| mmHg | 760 |
| psi | 14.696 |
Gas Laws
The ideal gas law, PV = nRT, describes the relationship between pressure (P), volume (V), number of moles (n), temperature (T), and the ideal gas constant (R).
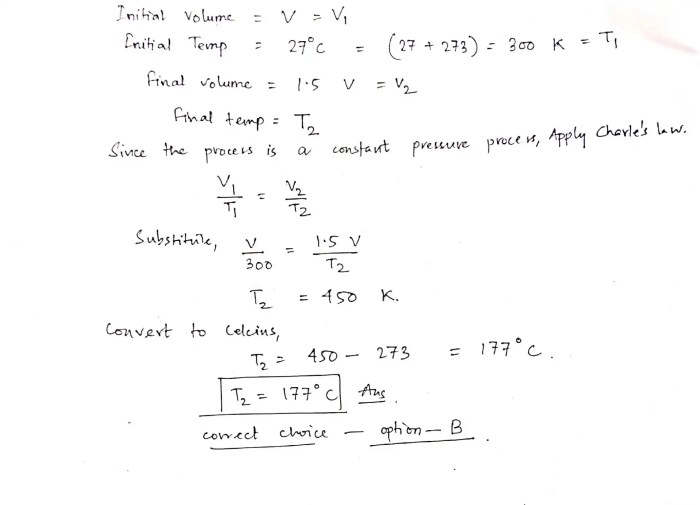
In this scenario, where pressure is 0.370 atm, we can use the ideal gas law to determine how pressure affects volume and temperature.
Units and Measurement
Using the correct units is essential in pressure measurements. The International System of Units (SI) defines the pascal (Pa) as the standard unit of pressure.
Pressure measurement devices, such as manometers and barometers, vary in precision and accuracy. Choosing the appropriate device for the specific application is crucial.
Applications of Pressure
Gas pressure finds applications in numerous fields:
- Scuba diving:Understanding pressure is vital for divers to calculate depth and prevent decompression sickness.
- Weather forecasting:Pressure gradients influence wind patterns and weather conditions.
Grasping pressure concepts enhances safety and performance in these applications.
Safety Considerations
Gas pressure can pose hazards if not handled properly.
Safety measures include:
- Using pressure-rated equipment
- Proper ventilation
- Training and certification for personnel handling pressurized systems
Questions Often Asked: A Gas Has A Pressure Of 0.370 Atm At 50.0
What is the significance of using the correct units in pressure measurements?
Using the correct units ensures consistency, accuracy, and comparability of measurements across different experiments and applications. It eliminates confusion and facilitates effective communication within the scientific community.
How does pressure affect the volume of a gas?
According to Boyle’s law, the volume of a gas is inversely proportional to its pressure at constant temperature. As pressure increases, the volume of the gas decreases, and vice versa.