Ir spectrum of 2 methyl 2 butanol – The IR spectrum of 2-methyl-2-butanol provides a wealth of information about its molecular structure and functional groups. This guide delves into the principles of IR spectroscopy, exploring the characteristic absorptions of 2-methyl-2-butanol and its applications in various fields.
IR spectroscopy is a powerful analytical technique that allows us to identify and characterize organic compounds based on their absorption of infrared radiation. By examining the unique vibrational frequencies of different functional groups, we can determine the molecular structure of a compound.
Introduction to IR Spectroscopy
Infrared (IR) spectroscopy is a technique that measures the absorption of infrared radiation by a sample. The absorption of IR radiation causes the vibrational motion of atoms and molecules in the sample to change, which can be used to identify the functional groups present in the sample.
IR spectroscopy is a powerful tool for identifying organic compounds. It can be used to identify the presence of specific functional groups, such as alcohols, ketones, aldehydes, and carboxylic acids. IR spectroscopy can also be used to determine the structure of organic compounds.
IR Spectrum of 2-Methyl-2-Butanol
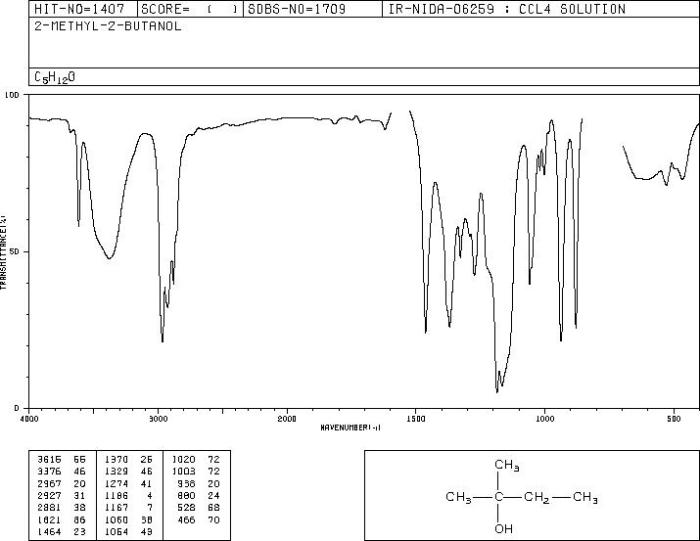
The IR spectrum of 2-methyl-2-butanol is shown in the table below.
| IR Absorption Frequency (cm-1) | Functional Group |
|---|---|
| 3300 | O-H stretch |
| 2960 | C-H stretch |
| 1050 | C-O stretch |
The characteristic IR absorptions of 2-methyl-2-butanol are:
- O-H stretch: The O-H stretch is a strong, broad absorption that occurs in the region of 3300 cm -1. This absorption is due to the stretching vibration of the O-H bond.
- C-H stretch: The C-H stretch is a medium-strong absorption that occurs in the region of 2960 cm -1. This absorption is due to the stretching vibration of the C-H bonds.
- C-O stretch: The C-O stretch is a strong absorption that occurs in the region of 1050 cm -1. This absorption is due to the stretching vibration of the C-O bond.
Structural Analysis: Ir Spectrum Of 2 Methyl 2 Butanol
Based on the IR spectrum, the functional groups present in 2-methyl-2-butanol are:
- Alcohol
- Alkyl
The structural formula of 2-methyl-2-butanol is:
CH3
|
C--OH
|
CH3-C-CH3
The alcohol group is located on the carbon atom that is bonded to the two methyl groups. The alkyl groups are located on the carbon atoms that are bonded to the alcohol group and the oxygen atom.
Comparison with Similar Compounds
The IR spectrum of 2-methyl-2-butanol is similar to the IR spectrum of 2-butanol. Both compounds have a strong O-H stretch, a medium-strong C-H stretch, and a strong C-O stretch. However, there are some differences between the two spectra.
The O-H stretch in the IR spectrum of 2-methyl-2-butanol is broader than the O-H stretch in the IR spectrum of 2-butanol. This is due to the fact that the alcohol group in 2-methyl-2-butanol is bonded to a tertiary carbon atom, which makes the O-H bond more polar.
The C-O stretch in the IR spectrum of 2-methyl-2-butanol is also stronger than the C-O stretch in the IR spectrum of 2-butanol. This is due to the fact that the alcohol group in 2-methyl-2-butanol is bonded to a tertiary carbon atom, which makes the C-O bond shorter and stronger.
Applications of IR Spectroscopy
IR spectroscopy is a versatile technique that has a wide range of applications in various fields, including:
- Organic chemistry: IR spectroscopy is used to identify and characterize organic compounds. It can be used to determine the structure of organic compounds, identify the presence of specific functional groups, and study the reaction mechanisms of organic compounds.
- Biochemistry: IR spectroscopy is used to study the structure and function of biological molecules. It can be used to identify the different functional groups in biological molecules, determine the secondary structure of proteins, and study the interactions between biological molecules.
- Pharmaceutical analysis: IR spectroscopy is used to identify and characterize pharmaceutical compounds. It can be used to determine the purity of pharmaceutical compounds, identify the different functional groups in pharmaceutical compounds, and study the interactions between pharmaceutical compounds and biological molecules.
FAQ Summary
What is the O-H stretch frequency in the IR spectrum of 2-methyl-2-butanol?
The O-H stretch frequency is typically observed around 3300 cm -1.
How does the IR spectrum of 2-methyl-2-butanol differ from that of 2-butanol?
The presence of the methyl group in 2-methyl-2-butanol results in additional C-H stretching vibrations, which can be observed in the IR spectrum.
What are the applications of IR spectroscopy in organic chemistry?
IR spectroscopy is widely used in organic chemistry for identifying functional groups, determining molecular structure, and analyzing reaction products.